Data- and knowledge-driven tools to accelerate Pharmaceutical Development
Data- and knowledge-driven tools to accelerate Pharmaceutical Development
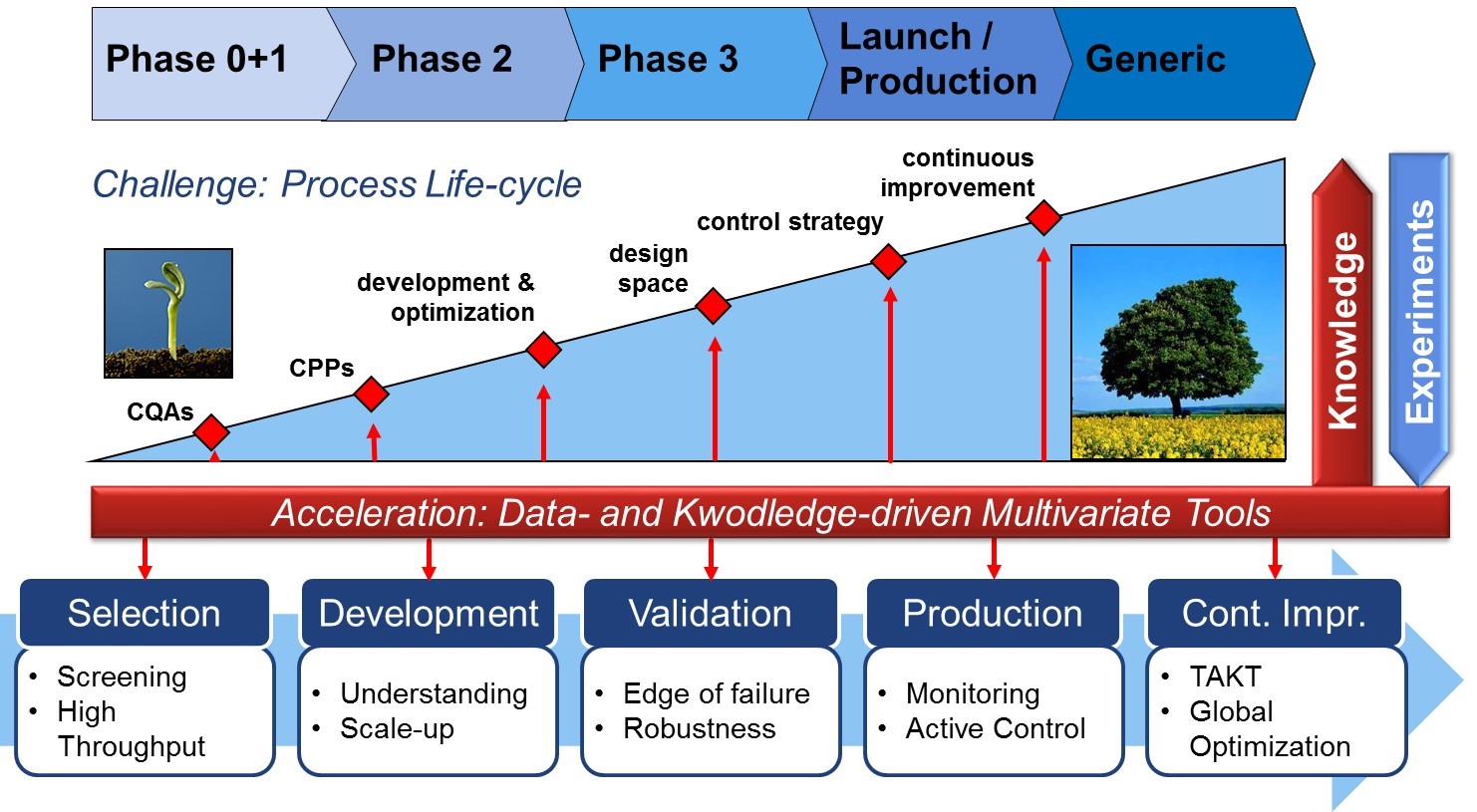
In the recent years, therapeutical proteins emerged to the leading 'biologics' with a cumulative turnover over 140 bln US $ in 2013. In order ensure the required product quality, the US Food and Drug Administration motivated the Quality by Design approach for the pharmaceutical process development. Critical quality attributes as well as critical process parameters shall be identified within the hundreds of potentially influential factors. Often the existing expertise and the experimental accessibility are limited so that, at first, the application of statistical analysis on the collected data can reveal very important, hidden patterns and correlations. Therefore, multivariate data-driven knowledge discovery is becoming an attractive tool in the pharmaceutical manufacturing, which can be applied at various stages in the drug lifecycle supporting the decision taking in the development team. Moreover, such models can be improved and guided by the existing knowledge providing an even more solid backbone for the decisions on process design. With this hybrid approach, eventually both, development costs and time to market can be considerably reduced.
Objectives
The objective is to show the versatile applicability of data-driven and hybrid tools at different stages of the pharmaceutical development. The advantages of their implementation shall be proven experimentally starting from efficient process screening at optimal conditions to multivariate model-based process control and scale-up.
Methodology
The focus of the project is on process development and optimization for the production of monoclonal antibodies (mAbs). Taking into account both characteristic stages, the upstream (expansion and fermentation) as well as the downstream (capture and purification), the effect of process parameters on the product quantity and quality is evaluated. Based on many case studies a data- and knowledge-driven engineering toolbox shall be developed tailored to the requirements of biopharmaceuticals. Fields of application include process screening (e.g. for biosimilars development), process classification and prediction, process monitoring using spectral data as well as process scale-up.
Contact Person: Michael Sokolov