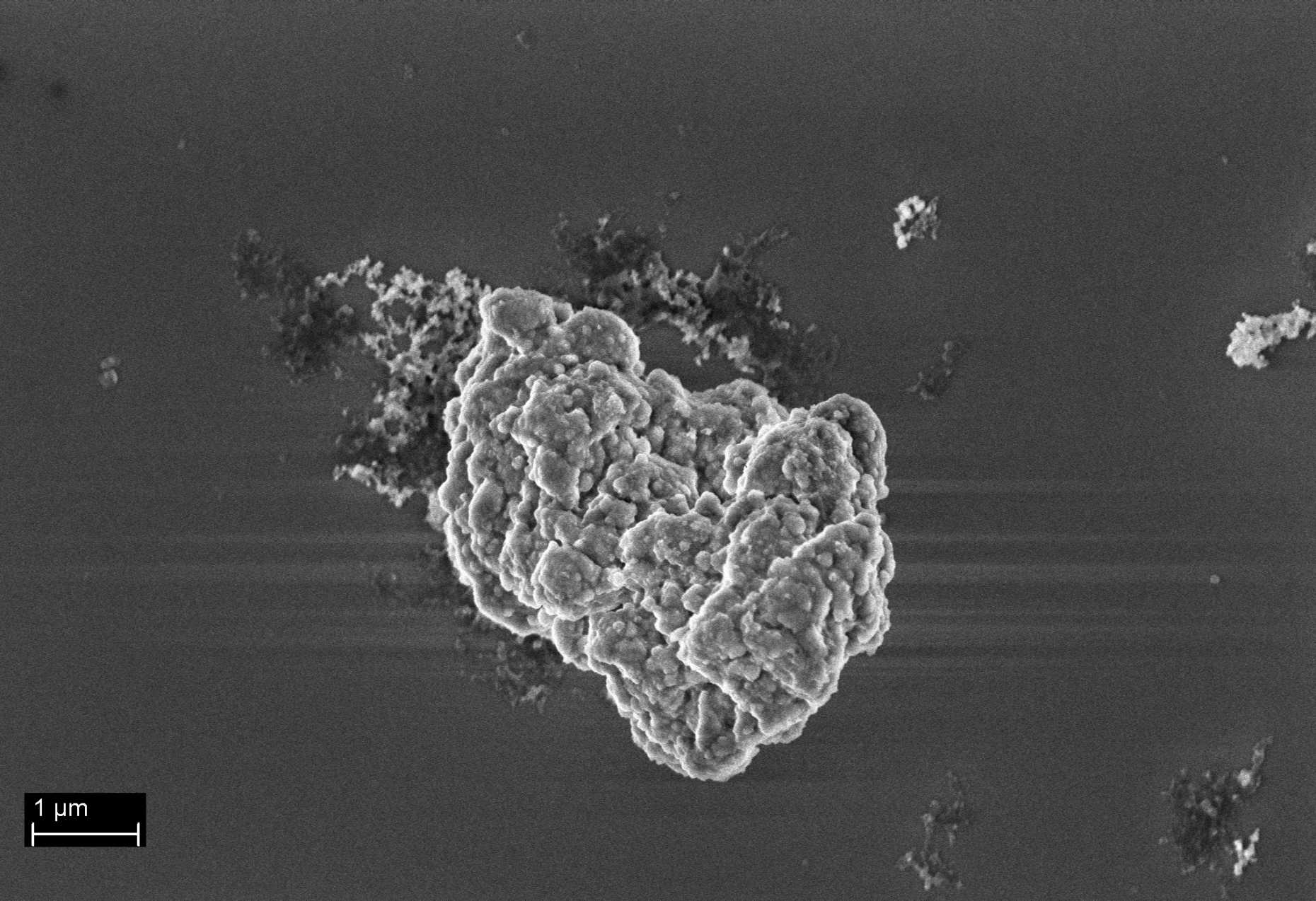
The control of size and morphology in the preparation of catalytic particles for Ziegler-Natta olefin polymerization
The Ziegler-Natta polymerization still remains sixty years after its discovery an important process for the production of polyolefins. In the polyethylene industry, the slurry polymerization process is dominating, whereas the gaseous monomer is lead through a suspension of heterogeneously supported Ziegler-Natta catalyst particles. Such a catalyst typically consists of an active species formed by a titanium compound, which is fixed on an Mg-containing support, to which an aluminum alkyl as co-catalyst is added.
During the polymerization reaction, catalyst particles expand under the action of the elongating polymer chains to give the final polymer particles, whereas the shape of the parent catalyst particles are retained. It is due to this replication phenomenon1 that the shape and particle size distribution of the final polymer reflects the initial shape and size of the parent catalyst particles. Due to the multiple active sites present on such a catalyst, by changing the reaction conditions, polyethylene with the broadest range of mechanical properties can be produced while using the same catalyst platform. To each of these processes, a target initial size prevails for the ideal catalyst, and therefore, controlling the catalyst particle size distribution is of high industrial importance.
This project aims at gaining knowledge about how an industrially relevant heterogeneous Ziegler-Natta catalyst particle forms. The focus is put on the evolution of the size of the particles during their synthesis by reactive precipitation.
Special efforts will be spent on assessing the effects of extremely high shear rates on the particle size of pre-synthesized particles in order to be able to infer a possible breakup mechanism. Finally, these shear rates will be applied during the reactive precipitation, and their effect on the interplay of aggregation, agglomeration and breakage of the precipitate will be evaluated.
The fundamental knowledge gained will then be used in order to devise a process enabling to control the final size of the catalyst and to minimize the width of the size distribution. Such a process will then be put into comparison with the standard precipitation method prevailing in industry.
Since the particles are destroyed by air and moisture, the handling of the catalyst has to be done using the Schlenk technique. In addition, all processes developed in the frame of this project must be under inert atmosphere. With respect to the characterization, a particle sizing strategy has to be especially devised in order to follow the growth of the particles during their synthesis through reactive precipitation as well as during high shear treatment.
Contact Person: Antoine Klaue